|
Anesthetic
Vaporizers
The purpose
of an anesthetic vaporizer is to produce a controlled and predictable
concentration of anesthetic vapor in the carrier gas passing
through the vaporizer.
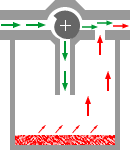
Most vaporizers
are of the plenum type, which consists of a vaporizing chamber
containing the liquid anesthetic, and a bypass. Gas passing
through the vaporizing chamber volatilizes the anesthetic and
is then mixed with the anesthetic-free gas bypassing the chamber,
the proportion of vapor-containing gas and bypass gas being
controlled by a tap.
Saturated
vapor pressure
The most important factor governing vaporizer design is the
saturated vapor pressure (SVP) of the anesthetic. SVP is a measure
of the volatility of the liquid anesthetic in the carrier gas:
after equilibration between the carrier gas and the liquid anesthetic,
the concentration of highly volatile anesthetics (e.g. isoflurane)
in the gas will be higher than that of poorly volatile anesthetics
(e.g. methoxyflurane).
Anesthetics
with a high SVP will require a smaller proportion of the total
gas flowing through the vaporizer to pass through the vaporizing
chamber to produce a given concentration than will anesthetics
with a low SVP. The following table shows the SVP of some anesthetics
at 20oC and the proportion of the total gas flow
required to pass through the vaporizing chamber to produce an
ouput concentration of 1% at a barometric pressure of 760 mmHg:
| |
SVP
@ 20oC
(mmHg)
|
Chamber
flow
Total flow |
| Halothane |
243 |
2.1
% |
| Isoflurane |
239 |
2.2
% |
| Enflurane |
175 |
3.4
% |
| Methoxyflurane |
23 |
32.0
% |
It follows
that it can be extremely dangerous to deliver anesthetics
from vaporizers for which they were not designed. A vaporizer
intended for use with methoxyflurane filled with isoflurane
and with the dial set to 1% would in fact be producing about
15 % isoflurane.
SVP and
temperature
Saturated vapor
pressure varies as a function of temperature:
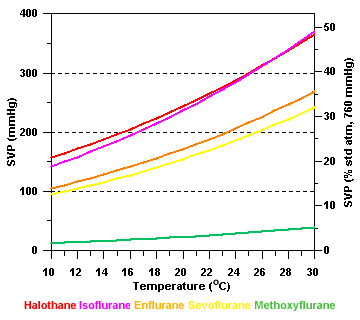
Unless some
means of compensation is employed, the vaporizer output will increase
as temperature rises, and vice versa.
Factors
affecting vaporizer output
- Flow
through the vaporizing chamber
Varying the proportion of gas passing through the vaporizing chamber
and bypass is the method by which vaporizer output is controlled.
- Efficiency
of vaporization
Vaporizers may incorporate a system of wicks and channels in the
vaporizing chamber to improve efficiency of vaporization and increase
the output concentration of anesthetic.
- Temperature
As temperature increases, the output of the vaporizer will increase,
unless some compensatory mechanism is used.
- Time
Vaporization causes the liquid anesthetic to cool since heat is
lost because of the latent heat of vaporization of the anesthetic.
Therefore, the output concentration will tend to fall over time.
- Gas flow
rate
Changes in carrier gas flow rate may affect vaporizer output by:
- Altering
the proportion of the total gas flow that passes through the
vaporizing chamber.
- Altering
the efficiency of vaporization. For example, at high flow
rates, the gas leaving the vaporizing chamber will tend to
be less saturated (since the gas spends less time in the chamber),
so the output of the vaporizer will tend to fall.
- Carrier
gas composition
The composition of the carrier gas may affect vaporizer output
by:
- Changes
in the viscosity and density of the gas mixture affecting
the proportion of the total flow that passes through the vaporizing
chamber
- Nitrous
oxide dissolving in the anesthetic, thus altering the effective
volume that passes through the vaporizing chamber.
- Ambient
pressure
Saturated vapor
pressure is solely a function of temperature. Therefore, if ambient
pressure is reduced, the (constant) SVP becomes a greater proportion
of the total (reduced) pressure, and the ouput concentration (in
volumes %) rises.
For example, a halothane vaporizer
calibrated at sea level and set to deliver 2% will produce about
2.7% halothane if used in Denver, Colorado.
Types of
vaporizer
Simple
vaporizers do not compensate for these effects. Their output
varies widely under different conditions.
Precision vaporizers incorporate
mechanisms to compensate for these effects. Their output is reasonably
constant over a wide range of conditions.
Low-resistance vaporizers have a low internal
resistance to gas flow and are designed to be used within the breathing
circuit, either in drawover apparatus or as in-circuit vaporizers
in the circle absorber system.
Simple
vaporizers 
|